Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
3 Minutes
Marco Capogrosso, PhD
Modern neurotechnologies hold promises to restore a variety of neural functions after neurological disorders. For example, spinal cord stimulation (SCS), initially developed for the treatment of refractory pain, is now finding great momentum as a tool to restore movement after spinal cord injury. Due to the ancestral attraction that the human animal has always had with movement in connection with the concept of life, these applications are easily appealing to the large public. But how much of this glitter actually translates into clinical practice?
Unfortunately, translation of exciting pre-clinical results obtained in research laboratories into clinical settings must undergo a slow but necessary validation process. Rigorous federal procedures regulate the submission of clinical testing of new technologies as well as cost-driven opportunities of industrial partners that can produce new devices. Therefore, economic and societal interests clash in a complicated maelstrom that the scientist is definitively unprepared to navigate.
In our group, we believe that working as scientists in a clinical department allows a direct interaction with clinical leaders in the field. This helps steer the day-to-day compass of scientific discovery towards realistic clinical goals.
For instance, we have been working on the development of SCS technology for the restoration of arm movement in people with arm paralysis. The proximity and continuous discussions with clinical investigators and the daily reality of clinical practice highlighted two critical factors that must be accounted for to translate our research to clinical practice:
These seemingly very practical points tamper with profound questions on the mechanisms of motor control and how electrical pulses modify the activity of spinal circuits to produce movements. Failure to address such questions can only lead to the definition of cumbersome and sub-optimal technical solutions likely relying on unpractical trial and error tuning procedures.
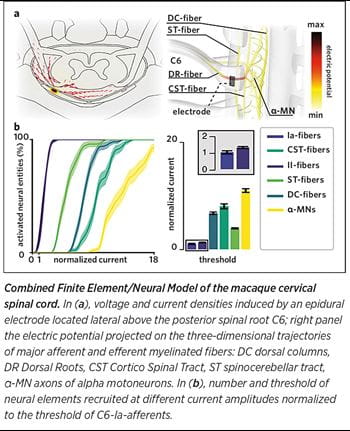 For this reason, we focused the activity in our laboratory on building a bridge between basic research in neural engineering and clinical stakeholders. We are studying the mechanisms of interaction between electrical stimulation and the spinal circuits to design simple, optimal technologies that leverage anatomical and physiological properties of neural circuits to maximize efficacy. In a recent pre-clinical study in monkeys, we combined computer simulations and electrophysiology to decipher the optimal neural target of SCS of the cervical spinal cord for the recovery of arm movements after spinal cord injury. We found that electrodes with lateral contacts can engage single dorsal roots and thus specific arm motoneurons via monosynaptic excitatory pathways with a high degree of reproducibility across subjects. We are now ready to translate these pre-clinical results in humans and verify if a simple technological solution can induce complex hand movements in people with arm paralysis.
For this reason, we focused the activity in our laboratory on building a bridge between basic research in neural engineering and clinical stakeholders. We are studying the mechanisms of interaction between electrical stimulation and the spinal circuits to design simple, optimal technologies that leverage anatomical and physiological properties of neural circuits to maximize efficacy. In a recent pre-clinical study in monkeys, we combined computer simulations and electrophysiology to decipher the optimal neural target of SCS of the cervical spinal cord for the recovery of arm movements after spinal cord injury. We found that electrodes with lateral contacts can engage single dorsal roots and thus specific arm motoneurons via monosynaptic excitatory pathways with a high degree of reproducibility across subjects. We are now ready to translate these pre-clinical results in humans and verify if a simple technological solution can induce complex hand movements in people with arm paralysis.
We strongly believe that basic research and engineering must go hand in hand with clinical research if we seriously want to tackle unsolved clinical challenges of neurological disorders and help patients with neurological deficits.
My post-doc mentor Grégoire Courtine used to randomly drop by my desk to ask, “What did you do today to improve the life of people with spinal cord injury?”
Let’s keep this question in mind during our daily life as basic researchers and mentors of new generations of investigators.