Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
2 Minutes
Several experts from the UPMC Department of Radiology, including Anish Ghodadra, MD, and Elliott Hammersley, collaborated to develop a solution for an electronic document control system for 3D printing at the point-of-care, which was published in 3D Printing in Medicine.
The rapid expansion and anticipated U.S Food and Drug Administration regulation of 3D printing at the point-of-care necessitates the creation of robust quality management systems. A critical component of any quality management system is a document control system for the organization, tracking, signature collection, and distribution of manufacturing documentation. While off-the-shelf solutions for document control exist, external programs are costly and come with network security concerns.
The internally developed, cost-effective solution is a hybrid document control system that they created by linking two commercially available platforms, Microsoft SharePoint and Adobe Sign, using a customized document approval workflow. The platform meets all Code of Federal Regulations Title 21, Part 11 guidances.
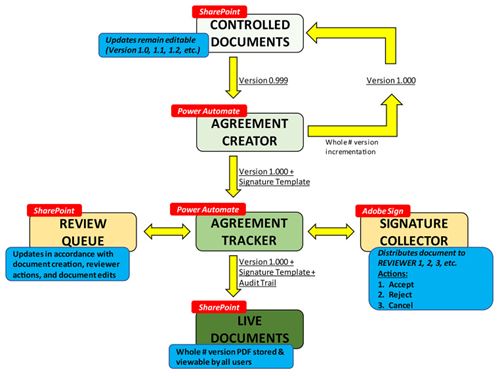
The hybrid solution for document control provides an affordable system for users to sort, manage, store, edit, and sign documents. The system can serve as a framework for other 3D printing programs to prepare for future U.S Food and Drug Administration regulation, improve the efficiency of 3D printing at the point-of-care, and enhance the quality of work produced by their respective program.
Read more on PubMed.
Other study authors include: