Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
4 Minutes
New work from the laboratory of Carlton M. Bates, MD, chief of the Division of Pediatric Nephrology at UPMC Children’s Hospital of Pittsburgh, shows that keratinocyte growth factor (KGF) works through AKT to mitigate bladder urothelial injury as a result of cyclophosphamide toxicity. Furthermore, direct AKT agonists are sufficient to afford protection against injury.
The study was initially published online ahead of print in the American Journal of Pathology in January 2022, and an image from the investigation will be featured on the cover of the journal’s April 2022 print edition.
Joining Dr. Bates on the investigation titled “AKT Signaling Downstream of KGF Is Necessary and Sufficient for Blocking Cyclophosphamide Bladder Injury,” were the authors Sridhar T. Narla, PhD, Daniel S. Bushnell, BS, and Joanne L. Duara, MD.
Cyclophosphamide (CP) is a chemotherapeutic agent used to treat various forms of cancer, nephrotic syndrome, and rheumatic diseases. However, CP has, among other toxicities, the potential to injure the bladder urothelium and cause hemorrhagic cystitis and in the long term, bladder cancer.
Previous work by Dr. Bates and colleagues showed that administering KGF, also known as fibroblast growth factor 7, prior to CP in mice blocked the widespread apoptosis within the bladder urothelium. Given KGF’s use in humans to block injury in other organs, this work showed its potential as a therapeutic agent that could be given in tandem with cyclophosphamide to mitigate its potential toxicities and bladder damage.
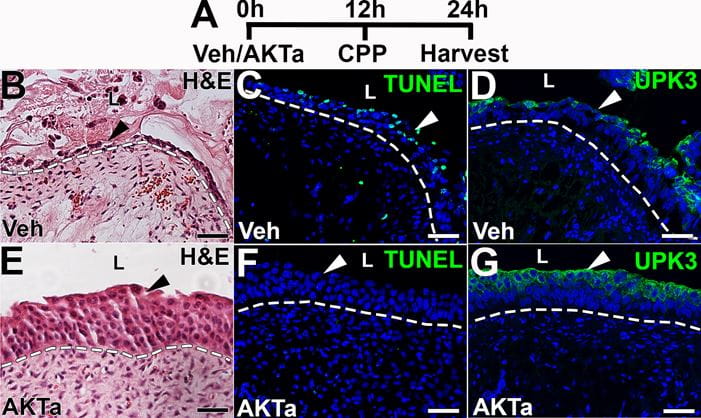
Representative images from cyclophosphamide injured mice showing urothelial cytoprotection and readouts of downstream targets of the AKT agonist (AKTa) 24 hours after treatment. A. Timeline of the experimental design (h = hour; Veh = vehicle for AKTa; CPP = cyclophosphamide). B-G. Images from injured vehicle-treated mice (B-D) and injured AKTa-treated mice (E-G) showing injury patterns. B. H&E image shows that vehicle-treated mice have significant urothelial injury (arrowhead), with sloughing and denuding cells. C. TUNEL staining (green) reveals many TUNEL+ apoptotic urothelial cells (arrowhead) in vehicle-treated mice. D. IF for UPK3 (green) shows regional losses of urothelial staining (arrowhead) in vehicle-treated mice. E. H&E image shows that AKTa-treated mice have largely intact urothelium (arrowhead). F. TUNEL staining (green) reveals virtually no urothelial apoptosis in AKTa-treated mice (arrowhead). G. IF for UPK3 (green) shows relatively intact expression in AKTa-treated mice (arrowhead), consistent with preservation of urothelial cell layers.
The new study expands upon this line of investigation and findings by showing how KGF blocks cyclophosphamide-induced urothelial injury. The study found that co-administration of an antagonist of AKT (a known signaling pathway downstream of KGF) blocked the ability of KGF to protect against cyclophosphamide-induced injury. Moreover, pre-treatment with a direct AKT agonist alone was sufficient to protect against cyclophosphamide-induced urothelial injury. Both KGF and the direct AKT agonists were shown to work by two further downstream targets – the B-cell lymphoma protein 2-associated agonist of cell death (BAD) and mammalian target of rapamycin complex (mTORC)-1. These two pathways themselves could be additional future therapeutic targets to mitigate bladder injury from cyclophosphamide.
Taken together, the findings of the new study point to the potential of the AKT pathway and further downstream pathways to be therapeutic targets for preventing bladder urothelial injury when cyclophosphamide is administered as an anticancer weapon. Indeed, the results of Dr. Bates and colleagues' study support targeting AKT to protect against epithelial injury in other tissues and clinical circumstances.
The laboratory is continuing its work investigating KGF and AKT in additional studies.
Read more about the research at the following link.
Narla ST, Bushnell DS, Duara JL, Bates CM. AKT Signaling Downstream of KGF is Necessary and Sufficient for Blocking Cyclophosphamide Bladder Injury. Am J Pathol. 2022 Jan 19: S0002-9440(00005-0). Online Ahead of Print.