Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
12 Minutes
Gillian Kane, BS
Research Assistant
University of Pittsburgh Department of Orthopaedic Surgery Biodynamics Laboratory
Clarissa LeVasseur, MS
Research Engineer
University of Pittsburgh Department of Orthopaedic Surgery Biodynamics Laboratory
Jonathan Hughes, MD
Sports Medicine Fellow
University of Pittsburgh Department of Orthopaedic Surgery
William Anderst, PhD
Assistant Professor of Orthopaedic Surgery, University of Pittsburgh Department of Orthopaedic Surgery
Director, Biodynamics Laboratory
Albert Lin, MD
Associate Professor of Orthopaedic Surgery, University of Pittsburgh Department of Orthopaedic Surgery
Associate Chief, Division of Sports Medicine
Rotator cuff tears (RCT) are the primary cause of shoulder disability in patients over the age of 60 and affect approximately 40% of this population.1 Each year, approximately 250,000 rotator cuff repairs are performed in the United States at a cost of $3 billion.1 Although traditional arthroscopic repairs often have satisfactory results, not all tears are reparable. Many tears larger than 5 cm, or tears involving two or more tendons with grade 3-4 fat infiltration, are massive irreparable tears,2,3 for which surgery cannot restore the anatomical footprint back onto the greater tuberosity of the humerus.3 The exact prevalence of irreparable RCT is unknown, but estimates ranging from 6.5% to 30% have been reported in the literature.4-8 Patients with irreparable RCT often exhibit debilitating symptoms, including impingement, weakness, and functional limitations believed to be caused by a lack of the stabilizing influence of the rotator cuff, resulting in superior migration of the humerus during shoulder abduction and elevation. In turn, this results in a lack of a stable fulcrum for normal deltoid function.9
The management of patients with irreparable RCT represents a significant challenge for orthopaedic surgeons. Traditionally, a variety of surgical options exist, including:
However, the ability to control glenohumeral (GH) kinematics, in particular superior migration of the humerus that leads to impingement and osseous changes in the shoulder, is questionable with many of the available nonarthroplasty treatment options.10 Additionally, the nonarthroplasty options have variable failure rates and outcomes in the literature.11-15 Many surgeons, therefore, utilize reverse total shoulder arthroplasty as a definitive treatment as it improves pain, function, and has good long-term clinical outcomes for appropriately selected patients. However, reverse total shoulder arthroplasty is classically indicated for older, low-demand patients with rotator cuff arthropathy and may not be an appropriate option for a young, active individual due to activity demands and concerns regarding implant longevity. As such, joint preservation and nonarthroplasty reconstructive techniques should be sought in this younger patient population.
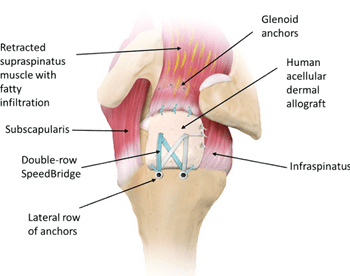
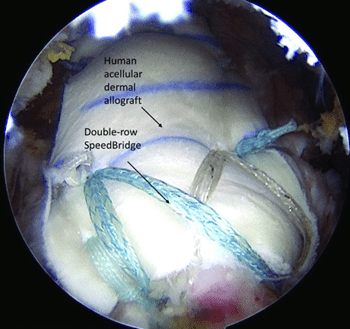 One viable treatment for massive irreparable RCT is an arthroscopic superior capsule reconstruction (SCR). SCR is a relatively new surgical method, first described by Mihata et al.,16 that involves placing a graft between the glenoid and humeral head, substituting for the deficient rotator cuff. A standard diagnostic arthroscopy is performed first to ensure the patient is a candidate for SCR, including assessing the articular cartilage and determining whether the rotator cuff tear truly is irreparable. If a reparable subscapularis tear is identified, this is repaired in conjunction with performing an intra-articular biceps tenodesis. Once the supraspinatus and/or infraspinatus are deemed irreparable, the SCR procedure is performed. Various grafts can be utilized, including a fascia lata autograft16 or acellular dermal allograft.17,18 Suture anchors are placed along the rotator cuff footprint on the humerus and the superior glenoid. These anchors are utilized to secure the graft in place. The graft is passed into the shoulder and secured in place on the glenoid and humerus, underneath the remaining rotator cuff tissue. The graft is sutured to the remaining rotator cuff medially and posteriorly for further stabilization.
One viable treatment for massive irreparable RCT is an arthroscopic superior capsule reconstruction (SCR). SCR is a relatively new surgical method, first described by Mihata et al.,16 that involves placing a graft between the glenoid and humeral head, substituting for the deficient rotator cuff. A standard diagnostic arthroscopy is performed first to ensure the patient is a candidate for SCR, including assessing the articular cartilage and determining whether the rotator cuff tear truly is irreparable. If a reparable subscapularis tear is identified, this is repaired in conjunction with performing an intra-articular biceps tenodesis. Once the supraspinatus and/or infraspinatus are deemed irreparable, the SCR procedure is performed. Various grafts can be utilized, including a fascia lata autograft16 or acellular dermal allograft.17,18 Suture anchors are placed along the rotator cuff footprint on the humerus and the superior glenoid. These anchors are utilized to secure the graft in place. The graft is passed into the shoulder and secured in place on the glenoid and humerus, underneath the remaining rotator cuff tissue. The graft is sutured to the remaining rotator cuff medially and posteriorly for further stabilization.
This technique is reserved for young, active patients with massive irreparable RCT of the supraspinatus and infraspinatus, minimal to no GH osteoarthritis, intact or reparable subscapularis tears, adequate deltoid function, and minimal proximal humeral head migration. In other words, patients well indicated for an SCR are those who demonstrate minimal rotator cuff arthropathy. These patients usually present with debilitating pain and/or unacceptable function and are poor candidates for reverse total shoulder arthroplasty due to longevity concerns of the prosthesis and the stresses placed on the prosthesis from high-activity demands. Once significant rotator cuff arthropathy, which includes severe superior migration of the humeral head or GH arthritis, has occurred (Hamada grade 3 or higher), SCR is no longer a viable treatment option.
SCR may provide the ability to restore GH kinematics, in particular superior migration of the humerus that leads to impingement and osseous changes in the shoulder. SCR has been shown to restore stability of the GH joint in cadavers,9 but due to the lack of in vivo data, the effectiveness of SCR at controlling in vivo humeral motion is unknown. So far, clinical results suggest improved range of motion and functional outcomes10 after superior capsule reconstruction. However, it is unclear exactly how SCR affects acromiohumeral distance (AHD), which is commonly used to describe the superior migration of the humerus relative to the scapula. Many believe an increase in AHD after surgery prevents continual abrasion of the graft between the humeral head and acromion. Additionally, restoration of normal AHD may represent improvement in superior joint stability and normal joint kinematics. However, no dynamic in vivo studies to date have addressed these controversies. Reported changes in static AHD following SCR are inconsistent, with some studies reporting an increase of 2.6 mm to 3.2 mm,17 while other studies reported no significant change.18 This data may be further complicated by comparisons between the use of autograft and allograft and varying differences in the thickness of the graft.17,18 Additionally, controversy still exists regarding whether SCR is an effective procedure for patients, as long-term outcome studies are not yet available and reported healing rates are variable.9,10,16-18 Most of the current literature on this topic can be found in technique papers or short-term outcomes studies from one of a few groups.9,10,16-18 Due to the lack of long-term outcome studies, lingering questions remain regarding which patients may benefit from this procedure, the longevity of the graft, and the overall effectiveness of the procedure. These questions regarding SCR and the lack of more precise, in vivo biodynamic studies serve as the scientific premise of our collaborative study between UPMC and the University of Pittsburgh Department of Orthopaedic Surgery Biodynamics Laboratory.
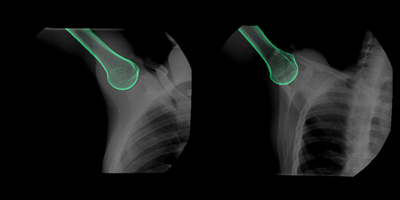 Novel Dynamic In Vivo Testing
Novel Dynamic In Vivo TestingAn ongoing collaborative study between UPMC and the University of Pittsburgh Department of Orthopaedic Surgery Biodynamics Laboratory is examining the effects of SCR on in vivo kinematics in 10 patients. Using biplane radiography, scapular and humeral motion is recorded before and one year after SCR during dynamic abduction, elbow rotation, and a functional hand to the head motion. 3D bone kinematics during these motions, accurate to 1 mm of translation and 1 degree of rotation, are then determined using a validated model-based tracking technique that matches a CT bone model to the synchronized biplane radiographs.
In addition to the 6° of freedom rotation and translational kinematics, subchondral bone measurements can be made throughout the motion, such as the distance between the acromion and humeral head and the relationship between the humeral head and the glenoid.
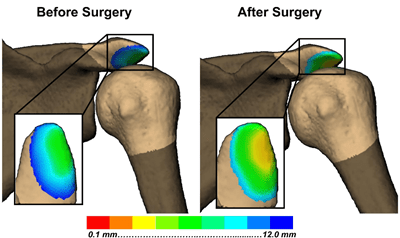
Furthermore, patient-reported outcome surveys (the American Shoulder and Elbow Surgeons (ASES) Shoulder Score; Western Ontario Rotator Cuff (WORC) Index; Disabilities of the Arm, Shoulder and Hand (DASH) Outcome Measure), and clinical strength and range of motion tests were collected presurgery (PRE), six months postsurgery, and one-year postsurgery (1YR-POST).
As a pilot study, this study serves as the beginning of a larger study for comparing SCR versus other joint preserving reconstructive procedures, with implications on cost of surgery, long-term outcomes, and the surgical risks associated with other surgeries (i.e., tendon transfer).
To date, nine patients have returned for postoperative testing. From this data, we focused on measuring acromiohumeral distance (AHD), scapulohumeral rhythm (SHR), maximum GH abduction, GH contact path, patient-reported outcomes (PROs), and graft healing. On average, AHD decreased from PRE to 1YR-POST surgery in both the static (PRE: 5.7 ± 1.2 mm, 1YR-POST: 4.6 ± 1.7, p = 0.11) and dynamic trials, but no statistical difference was identified (all p > 0.06). Additionally, as seen in Figure 1, the static AHD, which was taken with the arm around 20°of GH abduction, had higher average AHD than similar abduction angles during the dynamic motion. Therefore, the static AHD does not appear to represent AHD during dynamic motion.
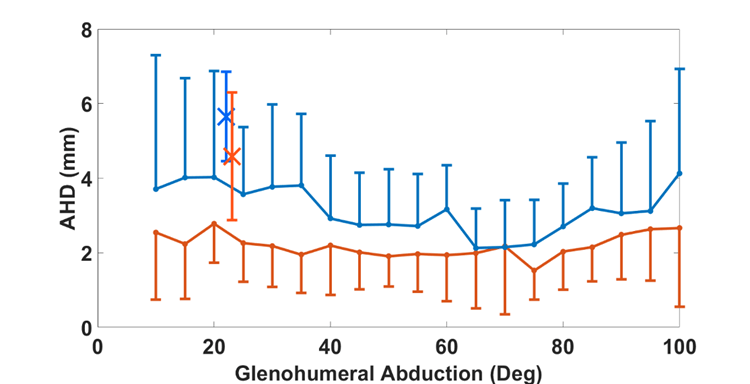
On average SHR did not change from before to after surgery (PRE: 1.9 ± 0.5, 1YR-POST: 1.6 ± 0.1, p = 0.18). However, there was a significant increase in maximum GH abduction seen in the difference in height of the Xs in Figure 2 (PRE: 77.2° ± 24.2°, 1YR-POST: 92.7° ± 12.4°, p = 0.03). Furthermore, in our observations we saw that there appeared to be two subgroups of people. The first group, subjects 2, 3, 6, and 8, seemed to reach higher GH abduction angles before surgery. These subjects appeared to have a fairly constant SHR from before to after surgery. Subjects 1, 4, 5, 7, and 9 exhibited much less GH abduction before surgery, in addition to a variety of changes in SHR from before to after surgery.
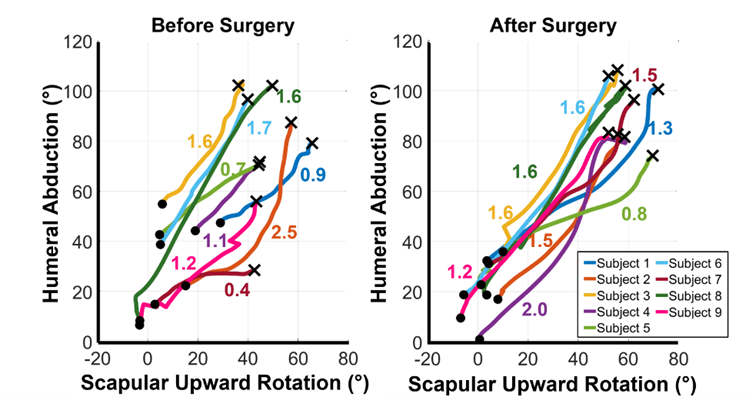
Overall, the GH contact path is quite variable with no obvious in vivo kinematics demonstrating a more centered contact path with shoulder abduction. Additionally, ASES, WORC, and DASH surveys were completed before surgery, six months after surgery, and one year after surgery. All patients improved beyond the minimal clinically important difference (MCID) for ASES, WORC, and DASH scores from PRE: 52 ± 25; 31 ± 14; and 44 ± 14 to 1YR-POST: 83 ± 14; 76 ± 11; and 13 ± 7 (all p = 0.008). Lastly, eight patients have received a one-year postoperative MRI to assess graft healing. Of those eight, three patients experienced full healing; four patients experienced partial healing defined as the graft partially healed on the glenoid or humerus, or partially healed at both sites; and one patient experienced graft failure defined as the graft not healed on the glenoid completely, on the humerus completely, or both.
In summary, our findings from our pilot study suggest that patients improve in functional motion and patient-reported outcomes following surgery, though from a kinematic standpoint we have not observed significant differences in AHD, SHR, or a predictable change in contact path following surgery. In addition, graft healing is variable, although seven of eight patients demonstrated either partial or full healing with only one patient demonstrating graft disruption and failure. In the future, in vivo kinematics will be critical to our understanding of whether SCR truly improves kinematics following surgery and whether SCR is a durable, joint-preserving procedure with favorable long-term results.
1. Amini MH, Ricchetti ET, Iannotti JP, Derwin KA. Rotator Cuff Repair: Challenges and Solutions. Orthop Res Rev. 2015; 7: 57-69.
2. Anley CM, Chan SK, Snow M. Arthroscopic Treatment Options for Irreparable Rotator Cuff Tears of the Shoulder. World J Orthop. 2014; 5(5): 557-565.
3. Gerber C, Wirth SH, Farshad M. Treatment Options for Massive Rotator Cuff Tears. J Shoulder Elbow Surg. 2011; 20(2): S20-S29.
4. Omid R, Lee B. Tendon Transfers for Irreparable Rotator Cuff Tears. J Am Acad Orthop Surg. 2013; 21(8): 492-501.
5. Moore DR, et al. Allograft Reconstruction for Massive, Irreparable Rotator Cuff Tears. Am J Sports Med. 2006; 34(3): 392-396.
6. Hirooka A, et al. Augmentation With a Gore-Tex Patch for Repair of Large Rotator Cuff Tears That Cannot Be Sutured. J Orthop Sci. 2002; 7(4): 451-456.
7. Ozaki J, et al. Reconstruction of Chronic Massive Rotator Cuff Tears With Synthetic Materials. Clin Orthop Relat Res. 1986; (202): 173-183.
8. Warner JJ. Management of Massive Irreparable Rotator Cuff Tears: The Role of Tendon Transfer. Instr Course Lect. 2001; 50: 63-71.
9. Mihata T, et al. Superior Capsule Reconstruction to Restore Superior Stability in Irreparable Rotator Cuff Tears: A Biomechanical Cadaveric Study. Am J Sports Med. 2012; 40(10): 2248-2255.
10. Mihata T, et al. Clinical Results of Arthroscopic Superior Capsule Reconstruction for Irreparable Rotator Cuff Tears. Arthroscopy. 2013; 29(3): 459-470.
11. Soler J, Gidwani S, Curtis M. Early Complications From the Use of Porcine Dermal Collagen Implants (Permacol) as Bridging Constructs in the Repair of Massive Rotator Cuff Tears. A Report of 4 Cases. Acta Orthop Belg. 2007; 73: 432-436.
12. Sclamberg S, Tibone J, Itamura J, Kasraeian S. Six Month Magnetic Resonance Imaging Follow-up of Large and Massive Rotator Cuff Repairs Reinforced With Porcine Small Intestinal Submucosa. J Shoulder Elbow Surg. 2004; 13: 538-541.
13. Moore D, Cain E, Schwartz M, Clancy WJ. Allograft Reconstruction for Massive, Irreparable Rotator Cuff Tears. Am J Sports Med. 2006; 24: 392-396.
14. Audenaert E, Van Nuffel J, Schepens A, Verhelst M, Verdonk R. Reconstruction of Massive Rotator Cuff Lesions With a Synthetic Interposition Graft: A Prospective Study of 41 Patients. Knee Surg Sport Traumatol Arthrosc. 2006; 14: 360-364.
15. Muench LN, Kia C, Williams AA, Avery III DM, Cote MP, Reed N, Arciero RA, Chandawarkar R, Mazzocca AD. High Clinical Failure Rate After Latissimus Dorsi Transfer for Revision Massive Rotator Cuff Tears. Arthroscopy. 2020; 36(1): 88-94.
16. Mihata T, Lee TQ, Watanabe C, et al. Clinical Results of Arthroscopic Superior Capsule Reconstruction for Irreparable Rotator Cuff Tears. Arthroscopy. 2013; 29: 459-470.
17. Pennington WT, Bartz BA, Pauli JM, Walker CE, Schmidt W. Arthroscopic Superior Capsular Reconstruction With Acellular Dermal Allograft for the Treatment of Massive Irreparable Rotator Cuff Tears: Short-Term Clinical Outcomes and the Radiographic Parameter of Superior Capsular Distance. Arthroscopy. 2018; 34(6): 1764-1773.
18. Denard PJ, Brady PC, Adams CR, Tokish JM, Burkhart SS. Preliminary Results of Arthroscopic Superior Capsule Reconstruction With Dermal Allograft. Arthroscopy. 2018; 34(1): 93-99.