Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
9 Minutes
The Heart Institute at UPMC Children’s Hospital of Pittsburgh has embarked on a multiyear, multifaceted expansion of the pediatric cardiac catheterization laboratory that will introduce new technologies for patient care, expand treatment options, and enhance UPMC Children’s research capabilities.
 Leading the effort is the new Director of the Cardiac Catheterization Laboratory (CCL) and Interventional Cardiology Service Bryan H. Goldstein, MD. Dr. Goldstein joined UPMC Children’s in December 2019 as the new director of the CCL. Dr. Goldstein takes over director duties for the CCL from Pediatric Cardiology Division Chief and Medical Director of the Heart Institute Jacqueline Kreutzer, MD, FAAC, FSCAI, who has been responsible for growing the program over the last decade to become a nationally and internationally recognized leader in pediatric cardiac catheterization procedures and research. Dr. Kreutzer will continue her catheterization practice and research but will be focusing more on expanding the academic and research programs of the entire Heart Institute at UPMC Children’s.
Leading the effort is the new Director of the Cardiac Catheterization Laboratory (CCL) and Interventional Cardiology Service Bryan H. Goldstein, MD. Dr. Goldstein joined UPMC Children’s in December 2019 as the new director of the CCL. Dr. Goldstein takes over director duties for the CCL from Pediatric Cardiology Division Chief and Medical Director of the Heart Institute Jacqueline Kreutzer, MD, FAAC, FSCAI, who has been responsible for growing the program over the last decade to become a nationally and internationally recognized leader in pediatric cardiac catheterization procedures and research. Dr. Kreutzer will continue her catheterization practice and research but will be focusing more on expanding the academic and research programs of the entire Heart Institute at UPMC Children’s.
“Under Dr. Kreutzer’s leadership, the CCL has markedly expanded its capabilities and affords patients some of the highest quality standards and procedural outcomes in the field. The success of the cardiac catheterization program at UPMC Children’s is one of the reasons our Heart Institute is now ranked #2 in the country by U.S. News & World Report,” says Mark Sevco, president of UPMC Children’s.
An Associate Professor of Pediatrics at the University of Pittsburgh School of Medicine, Dr. Goldstein most recently held appointments at Cincinnati Children’s Hospital, where he was the Associate Director of the Cardiac Catheterization Laboratory. Dr. Goldstein earned his medical degree at the Boston University School of Medicine, followed by pediatric residency training at Boston Children’s Hospital. Dr. Goldstein next completed fellowships in cardiology and interventional cardiology at the University of Michigan C.S. Mott Children’s Hospital in Ann Arbor.
In addition to his responsibilities at UPMC Children’s, Dr. Goldstein is co-founder and vice president of the Congenital Catheterization Research Collaborative (CCRC), a multicenter academic collaborative that fosters research and quality improvement efforts amongst a group of 10 congenital heart centers in the United States. The CCRC focuses on conducting outcomes research following surgical and transcatheter interventions for congenital heart disease. Upon his arrival, UPMC Children’s joined the CCRC as its tenth member.
“We are pleased to have Dr. Goldstein join the Heart Institute at UPMC Children’s and expand upon the stellar work that has propelled the Institute to the highest levels of patient care and recognition in the United States and internationally. Dr. Goldstein will continue to build upon our clinically outstanding program, and further develop our academic and research platforms to spur the next generation of advances in pediatric cardiology,” says Victor Morrel, MD, chief of Pediatric Cardiothoracic Surgery, Heart Institute co-director, and UPMC Children’s Surgeon-in-Chief.

CCL Leadership. L to R: Bryan Goldstein, MD; Sara Trucco, MD; Jacqueline Kreutzer, MD.
The two existing CCLs at UPMC Children’s are planned to undergo complete reconstruction and refitting with the latest technologies and equipment for cardiac catheterization procedures. In addition, a new third lab space will be constructed in collaboration with the Department of Radiology to house a hybrid MRI/catheterization suite that will allow for both cardiac MRI and cardiac catheterization procedures to be conducted simultaneously on two patients, or combined at the same time for patients in need of both catheterization and MRI.
“One of our goals is to conduct radiation-free cardiac catheterization with MR guidance for both diagnostic and interventional procedures. This field is in its infancy, but we anticipate that it will grow over time; getting in on the ground floor makes sense as a national leader in congenital heart disease management. However, this technology will not only allow for radiation-free procedures, it also will allow us to develop novel programs that rely upon both interventional techniques and cardiac MRI imaging data. We are preparing for the future, but that future is near,” says Dr. Goldstein.
One of the clinical interests this new technology will allow Dr. Goldstein and colleagues to explore includes lymphatic interventions, a more recently identified major source of pathology and morbidity in patients with complex congenital heart disease.
“Plans for a lymphatic intervention program are in progress. We know we will be advancing more into this field to better treat many of our patients,” says Dr. Goldstein.
The new imaging and intervention technologies will expand the Heart Institute’s ability to comprehensively manage patients with single ventricle circulation, for which there are some current limitations in terms of treatment approaches. The hybrid suite will significantly reduce those limitations in the near future. This project also will allow for the expansion of invasive electrophysiology services, such as complex arrhythmia ablations and implantation of pacemakers and defibrillators in the CCL.
For those patients with cyanotic congenital heart disease, keeping the patent ductus arteriosus (PDA) open after birth is of great importance. For patients with ductal-dependent pulmonary blood flow — including patients with Tetralogy of Fallot, pulmonary atresia, and many other heart defects — transcatheter PDA stent implantation is a viable alternative to Blalock-Taussig (BT) shunt placement performed through an open surgical procedure.
Dr. Goldstein brings extensive clinical experience in catheter-based PDA stent procedures, and it has been a significant area of research for the CCRC, with numerous studies published by the group on the procedure over the last several years. See PDA stent placement using the flip technique on the left. The patient is positioned backward compared to normal positioning to facilitate the procedure.
“The PDA stent procedure allows us to place a small coronary stent in the ductus arteriosus to maintain its patency — thereby allowing a patient to go home from the hospital — facilitating normal growth and bonding at home until the time of the next intervention when surgery would be performed. It affords the patient excellent palliation for their cyanotic congenital heart disease while also avoiding a major open-heart surgery as their first intervention in the high-risk neonatal period,” says Dr. Goldstein.
A common comorbidity in very preterm neonates is persistent PDA. Neonates delivered before 30-weeks’ gestation or who weigh less than 1500 grams at birth are at high risk for PDA. Having a PDA persist into postnatal life – in the setting of an otherwise healthy heart – can be significantly harmful to the premature infant, accelerating the development of chronic lung disease and increasing the risk of other complications, including pulmonary hypertension.
For decades, older children with PDA who require closure have been successfully treated in the catheterization laboratory. Until recently, the only safe and viable option for very preterm neonates was surgical PDA ligation performed through a thoracotomy incision. This invasive procedure was accompanied by substantial risks of postsurgical morbidity, which limited its use. Recently, techniques and equipment have evolved that facilitate the use of catheter-based techniques for closure of the preterm PDA, even in the extremely low birth weight infant.
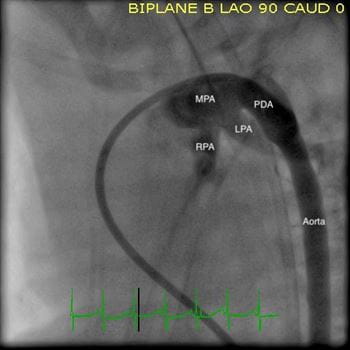
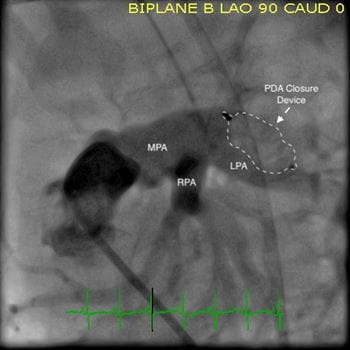
Angiograms from a PDA closure procedure in a preterm infant. Left: Baseline. Right: After closure. The patient was a 900-gram 24-week preterm infant. MPA: Main Pulmonary Artery; LPA: Left Pulmonary Artery; RPA: Right Pulmonary Artery; PDA: Patent Ductus Arteriosis.
Dr. Goldstein’s substantial clinical experience will allow UPMC Children’s to develop a robust PDA closure program in preterm neonates. The ability to offer this patient population access to a much less invasive procedure — with lower rates of complications and postprocedural morbidities in a catheter-based, short procedure — stands to greatly improve care in select patients for whom the procedure can benefit.
“We now have the tools and technology to transition most of these patients who require PDA ligation to transcatheter device closure via a short procedure. The first catheterization procedure that I performed after arriving at UPMC Children’s was a PDA closure on the smallest patient ever treated in the catheterization laboratory here — just under 800 grams,” says Dr. Goldstein.
Dr. Goldstein and colleagues are set to expand the academic development and research programs associated with the cardiac catheterization laboratory on three separate fronts. The first has been UPMC Children’s joining the CCRC network of high-impact congenital heart centers to take part in its multicenter studies. The hospital is also likely to join and contribute to other multicenter research collaboratives in the future. More information about the CCRC can be found in Petit CJ, et al. Comprehensive Comparative Outcomes in Children with Congenital Heart Disease: The Rationale for the Congenital Catheterization Research Collaborative. Congenital Heart Dis. 2019; 14: 341-349.
A second priority is to facilitate UPMC Children’s close collaboration with industry partners to conduct clinical trials on novel devices to treat various forms of congenital heart disease.
“Not only will we be able to contribute our significant resources and expertise to test some of these new devices, but it will also allow us to provide our patients greater access to emerging technologies that could afford life-altering and life-saving benefits,” says Dr. Goldstein.
Additionally, Dr. Goldstein and colleagues at the Heart Institute at UPMC Children’s will work on assessing the experiences, novel techniques, and programs related to catheterization procedures as a way to drive clinical innovation, be it through improvement in techniques, novel uses of existing technology, or the development of new platforms and devices.
"One area of excitement that I, personally, am looking forward to is collaborating with colleagues at the McGowan Institute for Regenerative Medicine and other local collaborators on potential device development and research,” says Dr. Goldstein. “I am honored to be surrounded by an incredible group of collaborators, clinicians, and resources here at UPMC Children’s. I look forward to building upon the exceptional catheterization program that Dr. Kreutzer and others have already propelled to the highest levels of clinical and research excellence.”
Interested in receiving specialty-specific information? Register for UPMC Physician Resources to get emerging research, clinical updates, and free CME straight to your inbox.