Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
11 Minutes
Jessica Reyes-Angel, MD
Fellow, Division of Pulmonary Medicine, Department of Pediatrics,
UPMC Children’s Hospital of Pittsburgh
Erick Forno, MD, MPH, ATSF
Assistant Professor, Division of Pulmonary Medicine, Department of Pediatrics,
UPMC Children’s Hospital of Pittsburgh
LF is a 9-year-old boy with a history of poorly controlled, severe persistent asthma, allergic rhinitis, and food allergies who presented with the chief complaint of drowsiness.
On the day of presentation, LF was seen in the Severe and Difficult-to-treat Asthma Clinic at UPMC Children’s Hospital of Pittsburgh. He was found to be drowsy, uncooperative, and hypotensive (blood pressure 87/62 mmHg), and he was immediately referred to the Emergency Department (ED) for further evaluation. His mother reported a 10-day history of drowsiness, headaches that worsened upon standing, decreased appetite, nausea, dizziness, and chills. She stated that his symptoms had started gradually but had progressively worsened over the past couple of days; no particular alleviating or exacerbating factors were identified. She denied fever, wheezing, shortness of breath, chest pain, emesis, abdominal pain, or diarrhea.
LF’s asthma treatment included high-dose fluticasone/salmeterol HFA (230/21 mcg, 2 puffs twice a day, for most of the past three years), tiotropium 2.5 mcg, 1 puff daily, and montelukast 5 mg daily. Despite these medications, he had received courses of oral prednisone almost monthly over the past year for acute asthma exacerbations. The last course had been 13 days prior, when he was prescribed 15 mg of prednisone (0.4 mg/kg/day) for five days. Because he did not improve with that regimen, he received an additional three-day course of 60 mg of prednisone per day (1.6 mg/kg/day), followed by a four-day taper (30 mg per day for two days, then 15 mg per day for two days), that was completed the day prior to his admission.
In the ED, the patient was afebrile, alert and cooperative, hydrated, and he did not have signs of bronchospasm. Due to the presenting symptoms in the clinic and the history of prolonged exposure to both inhaled and systemic steroids, acute adrenal crisis in the setting of chronically suppressed adrenal glands was suspected. Cortisol and ACTH levels were ordered, and hydrocortisone 2 mg/kg IV was administered. Following the hydrocortisone bolus, blood pressure normalized and dizziness resolved. Cortisol and ACTH levels, obtained prior to receiving hydrocortisone, were 4 mg/dL and 17 pg/mL, respectively. While detectable, these concentrations are not robust and were consistent with partial hypothalamic-pituitary-adrenal (HPA) axis suppression.
Because the HPA axis can take months to recover, a prolonged hydrocortisone taper was prescribed and the patient was referred to the UPMC Children’s Division of Pediatric Endocrinology, Diabetes and Metabolism. The patient’s mother reported that his symptoms resolved on the hydrocortisone.
A cosyntropin stimulation test was performed one month after he completed the taper and showed normal baseline and stimulated ACTH-cortisol responses, indicating that the patient no longer required stress-dose glucocorticoids.
In summary, LF is a 9-year-old boy with poorly controlled severe asthma who presented with drowsiness, headaches, decreased appetite, nausea, dizziness, chills, and hypotension in the setting of chronic exposure to high doses of inhaled corticosteroids, numerous courses of oral steroids over the past year, and a recent steroid course significantly above his physiologic dosing of 8-12 mg/m2/day of hydrocortisone (his last prednisone dose was equivalent to 49 mg/m2/day of hydrocortisone). In this situation, acute adrenal insufficiency should be suspected and treated with at least one parenteral glucocorticoid dose.
Asthma is the most prevalent chronic respiratory disease, affecting more than 330 million people worldwide. In the United States, approximately 7 million children have asthma.1 Glucocorticoids are the cornerstone for the long-term management of persistent asthma, since their anti-inflammatory effects reduce bronchial inflammation and hyperreactivity, resulting in better symptom control and decreased asthma morbidity and mortality. Current asthma guidelines recommend inhaled corticosteroids (ICS) as the first line of treatment in persistent asthma, and oral corticosteroids as adjuvants in the management of acute exacerbations.2
ICS at low to moderate doses and short-term oral corticosteroids courses are generally safe and not associated with clinically significant adverse effects.3 However, in the last two decades, there has been increased attention given to the effect of these medications on HPA axis in patients with asthma, particularly those receiving high doses of ICS or frequent systemic courses.
Adrenal insufficiency (AI) refers to an impaired secretion of adrenal hormones. It can result from primary dysfunction of the adrenal glands or from impairment of the HPA axis.4
Adrenal suppression (AS) refers to decreased endogenous cortisol production resulting from exposure to exogenous glucocorticoids.3
Adrenal crisis is a life-threatening episode of adrenal insufficiency characterized by severe hypotension and/or hypoglycemia that may lead to seizures and coma.
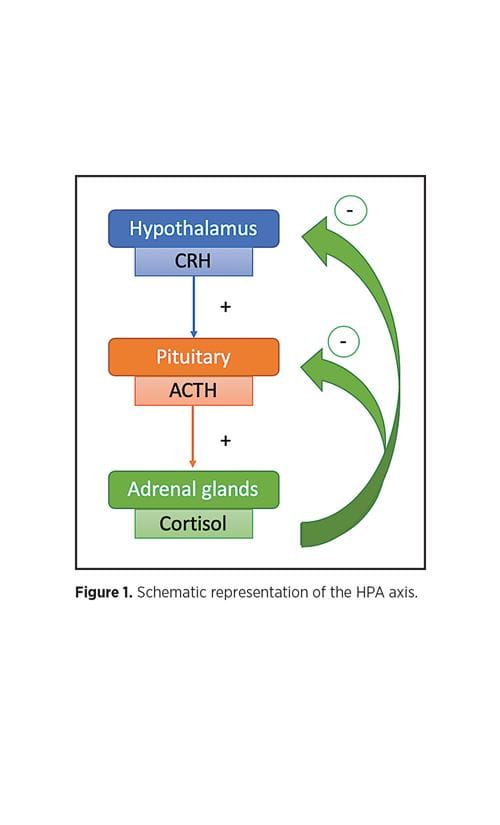
The HPA axis regulates the production of adrenal glucocorticoids.5 Neurons in the hypothalamus synthesize corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary to produce adreno-corticotropic hormone (ACTH), which in turn induces the adrenal cortex to secrete cortisol, the major endogenous glucocorticoid. Glucocorticoids, whether endogenous or exogenous, exert negative feedback at the pituitary and hypothalamus levels3 (Figure 1). Generally, the HPA axis recovers rapidly (days to weeks) after cessation of glucocorticoids, but recovery can take six to 12 months following prolonged exposure.4,6
Cortisol has multiple functions in the body. It stimulates hepatic gluconeogenesis and lipolysis, maintains serum glucose concentrations, maintains blood pressure, and regulates immune response.4 Cortisol production is critical during periods of physiological stress (e.g., infection, burns, surgery). Adrenal suppression secondary to exogenous glucocorticoid treatment can precipitate adrenal crisis and death.5,7
AS usually has an insidious presentation with nonspecific symptoms, such as fatigue, lethargy, weakness, nausea, abdominal pain, vomiting, weight loss, and headaches. For this reason, this disorder can often go unrecognized until an illness or severe injury precipitate an acute adrenal crisis.7 Because of the nonspecific symptoms, patients with AI should receive education and should carry a “steroid emergency card” that they can show to emergency care providers.5
Screening and diagnostic tests for AS include:
Extensive clinical experience with ICS over the last 30 years suggests that the risk of AI caused by ICS treatment alone is low. However, this risk may be more significant in patients receiving high-dose or prolonged ICS. Some studies have shown that up to 90% of patients who developed AI during ICS treatment were receiving doses equivalent to ≥ 1500 mg/day of budesonide.9,10
Additionally, several factors could precipitate HPA axis dysfunction in patients receiving lower doses of ICS, such as a small body size, the concomitant use of other forms of corticosteroids (particularly nasal sprays), or use of CYP3A4 liver enzyme inhibitors (e.g., some macrolides, antifungals, and antivirals) which could interfere with ICS metabolism.11
On the other hand, the risk for AS seems to vary among different types of ICS. Most studies have found a higher impact on adrenal function with fluticasone propionate, which has been attributed to its pharmaco-dynamic and pharmacokinetic properties (long half-life and high lipophilicity).10-12 Conversely, ciclesonide has shown a lower risk of AS, possibly because its active metabolite undergoes hepatic first-pass metabolism, minimizing its systemic bioactivity.12-14
Short-term OCS courses used in asthma exacerbations are normally considered to be safe6 and are clinically indicated for acute management in that setting. However, their side effects have not been extensively evaluated and their relative risks are unclear.15 Available evidence suggests that children who require multiple OCS courses per year may be at higher risk for developing adrenal insufficiency. While these children may appear to have intact adrenal function based on baseline morning cortisol, their stress response may be insufficient, and thus they may be at risk of an adrenal crisis when sick (i.e., with an asthma exacerbation). Some studies have shown that the risk of adrenal crisis doubles with each additional OCS course per year.16
Corticosteroids (inhaled and oral) are the cornerstone for the treatment of asthma, and the risk of AS is low in patients treated with ICS at recommended doses. However, health care providers must be aware of the risk of adrenal suppression in patients who receive high doses of ICS, prolonged ICS, or repeated courses of oral steroids. For this reason, consultation with an asthma specialist is strongly recommended if asthma control is poor or if treatment escalation is necessary.
Currently, there are no national guidelines for AS screening in children with asthma, and available evidence suggests that screening approaches vary widely. Based on this, the Divisions of Pulmonary Medicine, Allergy/Immunology, and Endocrinology at UPMC Children’s have joined efforts to develop a study that seeks to better understand the endocrine consequences of corticosteroid treatment (inhaled and systemic) in patients with asthma seen in our Difficult-to-Treat Asthma Clinic.
The authors thank Ally Larkin, MD (Allergy/Immunology), and Selma Witchel, MD (Endocrinology), for their help with this article.
1. CDC. Most Recent Asthma Data | CDC. Centers for Disease Control and Prevention.
2. Global Initiative for Asthma. 2019 GINA Report: Global Strategy for Asthma Management and Prevention 2019; 2018. doi:10.1002/uog.8947.
3. Ahmet A, Kim H, Spier S. Adrenal Suppression: A Practical Guide to the Screening and Management of This Under-Recognized Complication of Inhaled Corticosteroid
Therapy. Allergy, Asthma Clin Immunol. 2011; doi:10.1186/1710-1492-7-13.
4. Shulman DI, Palmert MR, Kemp SF. Adrenal Insufficiency: Still a Cause of Morbidity and Death in Childhood. Pediatrics. 2007; doi:10.1542/peds.2006-1612.
5. Bowden SA, Connolly AM, Kinnett K, Zeitler PS. Management of Adrenal Insufficiency Risk After Long-Term Systemic Glucocorticoid Therapy in Duchenne Muscular Dystrophy: Clinical Practice Recommendations. J Neuromuscul Dis. 2019; doi:10.3233/JND-180346.
6. Ducharme FM, Chabot G, Polychronakos C, Glorieux F, Mazer B. Safety Profile of Frequent Short Courses of Oral Glucocorticoids in Acute Pediatric Asthma: Impact on Bone Metabolism, Bone Density, and Adrenal Function. Pediatrics. 2003; doi:10.1542/peds.111.2.376.
7. Bowden SA, Henry R. Pediatric Adrenal Insufficiency: Diagnosis, Management, and New Therapies. Int J Pediatr. 2018; doi:10.1155/2018/1739831.
8. Kapadia CR, Nebesio TD, Myers SE, et al. Endocrine Effects of Inhaled Corticosteroids in Children. JAMA Pediatr. 2016; doi:10.1001/jamapediatrics.2015.3526.
9. Fitzgerald D, Van Asperen P, Mellis C, Honner M, Smith L, Ambler G. Fluticasone Propionate 750 mg/day Versus Beclomethasone Dipropionate 1500 mg/day: Comparison of Efficacy and Adrenal Function in Paediatric Asthma. Thorax. 1998; doi:10.1136/thx.53.8.656.
10. Lipworth BJ. Systemic Adverse Effects of Inhaled Corticosteroid Therapy: A Systematic Review and Meta-Analysis. Arch Intern Med. 1999; doi:10.1001/archinte.159.9.941.
11. Sannarangappa V, Jalleh R. Inhaled Corticosteroids and Secondary Adrenal Insufficiency. Open Respir Med J. 2015; doi:10.2174/1874306401408010093.
12. Todd GRG, Acerini CL, Ross-Russell R, Zahra S, Warner JT, McCance D. Survey of Adrenal Crisis Associated With Inhaled Corticosteroids in the United Kingdom. Arch Dis Child. 2002; doi:10.1136/adc.87.6.457.
13. Weinbrenner A, Hüneke D, Zschiesche M, et al. Circadian Rhythm of Serum Cortisol After Repeated Inhalation of the New Topical Steroid Ciclesonide. J Clin Endocrinol Metab. 2002; doi:10.1210/jcem.87.5.8447.
14. Postma DS, Sevette C, Martinat Y, Schlösser N, Aumann J, Kafé H. Treatment of Asthma by the Inhaled Corticosteroid Ciclesonide Given Either in the Morning or Evening. Eur Respir J. 2001; doi:10.1183/09031936.01.00099701.
15. Aljebab F, Choonara I, Conroy S. Systematic Review of the Toxicity of Short-Course Oral Corticosteroids in Children. Arch Dis Child. 2016; doi:10.1136/archdischild-2015-309522.
16. Mortimer KJ, Tata LJ, Smith CJP, et al. Oral and Inhaled Corticosteroids and Adrenal Insufficiency: A Case-control Study. Thorax. 2006; doi:10.1136/thx.2005.052456.