Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
8 Minutes
As often occurs in the process of conducting scientific research, unexpected discoveries during experiments can lead to new avenues of investigation. Sometimes these tangents lead to revolutionary and transformative scientific advances. Sometimes they go nowhere; sometimes, they hold promise but have to wait for their time in the spotlight.
Yaacov Barak, PhD, knows a little bit about these ups and downs of the scientific research process. Dr. Barak is an associate professor in the Department of Obstetrics, Gynecology, and Reproductive Sciences at the University of Pittsburgh School of Medicine, and a researcher at the Magee-Womens Research Institute, where he is invested in two primary avenues of research: placental development and its role in influencing fetal heart development, and adipocyte death in the context of obesity and type 2 diabetes. Both of these research areas have direct ties to his laboratory’s past and current molecular research of the transcription factors peroxisome proliferator-activated receptors gamma and delta (PPARγ and PPARδ, respectively).
A little more than 20 years ago, research findings by Dr. Barak and colleagues, who at the time were working on animal model research related to PPARγ and δ and their relationship to fat cells, diabetes, and glucose metabolism, uncovered something unforeseen in their murine models.
In 1999, Dr. Barak and colleagues published a paper in the journal Molecular Cell, showing that knockout of PPARγ disrupted placental vascularization as a result of dysregulated trophoblast tissue. In turn, the defects in the vasculature of the placenta led to fetal heart defects characterized by thinning of the myocardium.
“As we uncovered, PPARγ is crucial for placental development and is only expressed in the placenta. PPARγ is not expressed in the fetal heart. Therefore, we surmised, and then have proven over time and through additional experiments, the placental defects arising from the PPARγ knockout were leading to these severe defects in heart development. When we corrected PPARγ in the placenta, and only in the placenta, the fetal heart defects retreated, and normal development was restored,” says Dr. Barak.
The research would have to wait, though, to continue its advance.
“Our data, though definitive, were not able to gain immediate acceptance. Continued funding was problematic, as a consequence, so the research was put away with the hope of coming back to it later. The timing was just not right for it back then,” says Dr. Barak.
In the intervening years since Dr. Barak published his initial research, different groups examining at least six distinct gene knockouts in mice found similar connections between placental defects and congenital heart defects.
“In each case, placental defects also led to heart defects that could be corrected by rescuing the placental defect, showing that our earlier finding may not have been a flash in the pan,” says Dr. Barak. “Then, in the last five or six years, the research has begun to attract attention in the clinical arena. Other groups began pursuing a similar hypothesis by mining human birth defect records, for the most part unaware of what we found 20 years ago.”
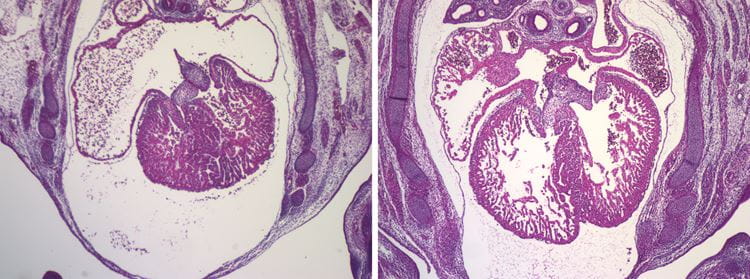
Hearts of two mouse embryos that share the same mutation. Left: As is. Right: The gene was corrected just in the placenta, proving that the heart defect is exclusively the result of placental malfunction.
“Cumulatively, what we and others have now shown is that there is a new developmental principle at play: placental defects can lead to congenital heart defects. Now the real work begins. We must build upon this foundation and carry forward the research — in our animal model studies and looking for similar traits in human patients.”
When the Magee-Womens Research Institute announced its inaugural research summit that was held in October 2018, it also announced the creation of the Magee Prize — a juried research award of $1 million to support transformative, innovative women’s health research.
Dr. Barak took his placenta/heart research off the proverbial shelf and assembled a proposal with a team of expert collaborators to rekindle this line of study and advance the mechanistic understanding of the molecular and cellular drivers of the placenta/heart defect associations his research has previously uncovered. Collaborating with Dr. Barak on this research are Myriam Hemberger, PhD, from the University of Calgary in Alberta, Canada, who studies the biology of the placenta, and Henry Sucov, PhD, from the Medical University of South Carolina in Charleston, who investigates pathways and molecular processes of cardiomyocyte proliferation and regeneration. Their proposal was one of 26 entries submitted to compete for the inaugural Magee Prize.
“Dr. Hemberger’s specialty is in the molecular biology of the trophoblast, and her past work that surveyed an unbiassed collection of more than a hundred embryonic-lethal murine knockout models found that placental defects were extremely prevalent and often coincided with heart defects. Dr. Sucov is an expert in cardiomyocyte development and regeneration, and has previously generated and characterized Rxra-null mice, which comprise one of the models we are currently using to examine the placental basis of congenital heart disease. Our team is well-positioned to investigate the mechanistic basis of placental defect-driven congenital heart disease in our model systems and start to see if these principles apply to the clinic. That is to say: What are the similarities and differences in newborns with congenital heart defects? I could not have asked for a more experienced and collaborative team to work with on this exciting project,” says Dr. Barak.
The 2018 Magee Prize, awarded to Dr. Barak and his two colleagues, funds a project with two specific aims related to the broader topic of the placenta/heart axis. The first aim of the study is to develop and characterize robust mouse models of placental-driven congenital heart disease, and in these models, to identify the precise types of associated defects in both tissues.
“These models will need to mimic congenital heart disease in human newborns more closely in terms of both the stage and defect type. They also need to be fully penetrant such that they are uniform and express the full-blown phenotype in every mutant embryo while exhibiting minimal pleiotropic or confounding phenotypes that originate outside of the placenta,” says Dr. Barak. “The creation of these models is a taxing endeavor but crucial to our studies.
The second aim of the study involves defining the mechanistic links between placental and heart development. To accomplish this aim, it is necessary to elucidate the pathways and biological processes that underpin a placental origin of congenital heart disease.
“Identifying the growth factors and signaling pathways at play between the placenta and the developing heart through transcriptomics, and uncovering placenta-produced hormones in our various models, will be the first steps in identifying some of the mechanistic links we seek. Based on incidental clues, we are also interested in the potential role that placental oxygen, or lack thereof, may play in this developmental axis, and will be assessing aberrations in the hypoxia response pathways of both tissues in our models,” says Dr. Barak.
These studies may one day lead to a fuller appreciation of the link between specific types of placental abnormalities and certain congenital heart defects. The placenta/heart axis may be perturbed by both genetic influences, as found in mice, and potentially also maternal physiology and environmental factors. The hope is that this work will form a foundation for future clinical studies that could uncover biomarkers of potential congenital heart disease related to placental defects, and one day lead to earlier detection, and ultimately therapy, of these disorders.
Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, Koder A, Evans RM. PPARγ Is Required for Placental, Cardiac, and Adipose Tissue Development. Mol Cell. 1999; 4(4): 585-595.
Sucov HM, Dyson E, Gumeringer CL, Price J, Chien KR, Evans RM. RXR Alpha Mutant Mice Establish a Genetic Basis for Vitamin A Signaling in Heart Morphogenesis. Genes Dev. 1994; 8: 1007-1018.
Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, While JK, Galli A, Tuck E, Ryder E, Gleeson D, Wardle-Jones H, Geyer S, Smith JC, Robertson E, Adams DJ, Weninger WJ, Mohun T and Hemberger M. Placentation Defects Are Highly Prevalent in Embryonic Lethal Mouse Mutants. Nature. 2018; 555: 463-468.
Barak Y, Sadovsky Y, Shalom-Barak T. PPAR Signaling in Placental Development and Function. PPAR Res. 2008; 2008: 142082.
Barak Y, Hemberger M, Sucov HM. 2019. Phases and Mechanisms of Embryonic Cardiomyocyte Proliferation and Ventricular Wall Morphogenesis. Pediatr Cardiol. 40(7): 1359-1366. Erratum in Pediatr Cardiol. 41(1): 220.
Interested in receiving specialty-specific information? Register for UPMC Physician Resources to get emerging research, clinical updates, and free CME straight to your inbox.