Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
3 Minutes
Pascal O. Zinn, MD, PhD
The current glioblastoma multiforme (GBM) treatment paradigm remains static in the face of this rapidly, and ever-evolving, niche-rich type of brain cancer. Irrespective of our expanding knowledge of GBM, prognosis and survival rates have only minimally improved over the last two decades. Currently, the median survival rates following surgical resection and chemoradiation are less than two years in 97% of patients. Moreover, median survival rate for patients who undergo supportive treatment without substantial medical or surgical interventions is only four months.
 A number of factors are responsible for such grim prognoses. The most significant of those are thought to be rapid growth, a heterogeneous cellular micro-environment, and the diverse mutational landscape of GBM promoting glioma stem cell propagation and a highly invasive nature of cellular spread precluding complete surgical resection. Given the uniform failure of current therapy facing off against this “multiform” disease, it urges us to explore more individualized biological and disease adaptable GBM treatment paradigms to address each patient’s unique type of cancer in a hyper-personalized fashion over time.
A number of factors are responsible for such grim prognoses. The most significant of those are thought to be rapid growth, a heterogeneous cellular micro-environment, and the diverse mutational landscape of GBM promoting glioma stem cell propagation and a highly invasive nature of cellular spread precluding complete surgical resection. Given the uniform failure of current therapy facing off against this “multiform” disease, it urges us to explore more individualized biological and disease adaptable GBM treatment paradigms to address each patient’s unique type of cancer in a hyper-personalized fashion over time.
This type of therapy is based on targeting specific molecular structures within GBM cells and hence downstream signaling pathways. Other examples of possible targets in GBM are angiogenesis, epigenetic modifiers (e.g., DNA methylation), and immune modulation. Such therapies can be tailored to each GBM patient and the cancer’s unique molecular profile.
The relationship between pharmacotherapy including chemotherapy and the chromosomal characterization of gliomas has been established. Pharmacogenomics is becoming an integral part in GBM post-surgical management. Currently, MGMT is used as a genetic marker that can predict temozolomide response and prognosis in general.
In vitro brain modeling has become an expanding field of research since the introduction of humanoid brain organoids in 2013. While most published brain organoid research describe the application of organoid technology in developmental and neurodegenerative disorders, few studies have illustrated application of such technology in GBM research by creating a GBM organoid model — either by coculturing GBM stem cells with induced pluripotent stem cells or by means of genetic engineering inducing de novo formation of cancer within the organoid.
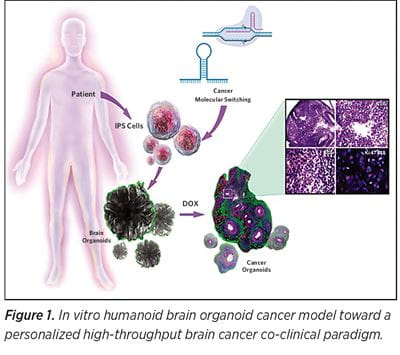
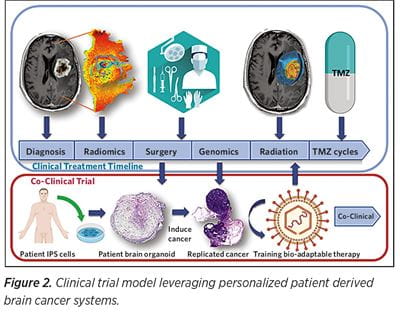 Despite all efforts, no personalized GBM treatment has been clinically implemented to date. The need for rapid and cost efficient personalized in vitro GBM therapy trials is apparent. In vitro humanoid brain organoid cancer models are the most apparent, robust, and realistic choice toward a personalized high-throughput brain cancer co-clinical paradigm (See Figure 1). We envision a patient-derived 3D in vitro cancer model system, recapitulating each patient GBM as well as serving as a bio-factory used to test and train different therapeutic agents (e.g. oncolytic viruses). At the University of Pittsburgh Department of Neurosurgery and the UPMC Hillman Cancer Center, a promising co-clinical trial model (Figure 2) is being developed that envisions leveraging personalized patient derived brain cancer systems to serve as therapy training avatars to boost tropism of oncolytic virus therapy.
Despite all efforts, no personalized GBM treatment has been clinically implemented to date. The need for rapid and cost efficient personalized in vitro GBM therapy trials is apparent. In vitro humanoid brain organoid cancer models are the most apparent, robust, and realistic choice toward a personalized high-throughput brain cancer co-clinical paradigm (See Figure 1). We envision a patient-derived 3D in vitro cancer model system, recapitulating each patient GBM as well as serving as a bio-factory used to test and train different therapeutic agents (e.g. oncolytic viruses). At the University of Pittsburgh Department of Neurosurgery and the UPMC Hillman Cancer Center, a promising co-clinical trial model (Figure 2) is being developed that envisions leveraging personalized patient derived brain cancer systems to serve as therapy training avatars to boost tropism of oncolytic virus therapy.
This model is a bio-adaptable therapy that continuously evolves to address the ever-evolving cancer landscape and anticipate cancer mutations in the model system before actually happening in the patient setting — a “minority report” of cancer therapy.