Password Reset
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
Forgot your password? Enter the email address you used to create your account to initiate a password reset.
9 Minutes
Timothy C. Wong, MD, UPMC Hypertrophic Cardiomyopathy Center
Therapy to reduce symptoms associated with severe left ventricular outflow tract (LVOT) obstruction represents a major facet of care for many patients with hypertrophic cardiomyopathy (HCM), a rare condition in which the myocardium thickens without any detectable systemic cause.1 Approximately two-thirds of HCM patients demonstrate resting, or provokable left ventricular outflow obstruction to normal blood flow from the heart, which may lead to symptoms including dyspnea on exertion, chest pain, or even syncope. Medications including beta-blockers and non-dihydropyridine calcium channel blockers, as well as disopyramide and others under development,2 are often able to reduce the outflow tract gradient and improve symptoms. However, for patients with severe obstruction and symptoms refractory to aggressive medical therapy, invasive septal reduction therapy is indicated to directly target the obstruction by thinning hypertrophied septal tissue and/or repairing an abnormal mitral valve apparatus.1
Two approaches represent the most common techniques employed to reduce the amount of hypertrophied myocardium impinging on the outflow tract: open heart surgery for septal myectomy and cardiac catheterization for alcohol septal ablation. Septal myectomy surgery to excise myocardial tissue is considered a “gold standard” invasive therapy for relief of outflow obstruction, although a minimally invasive procedure termed alcohol septal ablation is emerging as an alternative to open heart surgery in appropriately selected patients.
Dating back to 1964, septal myectomy has been performed under cardiopulmonary bypass and involves establishing access to the left ventricular cavity via aortotomy (or other approaches) and cutting away hypertrophied myocardium. This technique also permits repair of the mitral valve apparatus in specific cases where redundant mitral valve tissue might cause residual obstruction. Risks of the procedure include complications from cardiopulmonary bypass as well as inadvertent ventricular septal defects, along with an approximately seven percent risk of requiring permanent pacemaker implantation due to heart block.3
Alcohol septal ablation represents a minimally invasive alternative to open heart surgery for relief of outflow obstruction. First introduced in 1994, the procedure entails selecting an appropriate septal perforator artery supplying the hypertrophied myocardium most responsible for the obstruction, isolating the artery via an over-the-wire balloon, and infusing ethanol into the artery to induce myocardial infarction. Over time, the region of infarction thins as myocyte necrosis and replacement fibrosis occur, thereby relieving the outflow obstruction. Risks of the procedure include complications such as ventricular septal defects and complete heart block; recent series report a permanent pacemaker implantation risk of approximately 11 percent.4
A 79-year-old woman was referred to the UPMC Hypertrophic Cardiomyopathy Center for further management of HCM with severe LVOT obstruction. Her history was notable for well-controlled hyper-tension, gastroesophageal reflux disease, extensive prior smoking history with emphysema, dyslipidemia, and bladder cancer without evidence of disease. Her symptoms consisted of worsening dyspnea on exertion in the setting of stable lung disease.
Echocardiography revealed asymmetric septal hypertrophy with a basal anterior septal measurement of 1.8 cm, severely elevated peak LVOT gradient of 77 mm Hg, and moderate mitral regurgitation due to systolic anterior motion of the mitral valve with mitral annular calcification. (Figure 1, panels A, B, C) Cardiac MRI with contrast demonstrated only mild nonischemic fibrosis within the hypertrophied myocardium. (Figure 1, panel D) Given her worsening symptoms despite maximal medical therapy of beta-blockade, and contraindications to disopyramide therapy, the patient was counseled regarding septal reduction therapy. Based on the patient’s smoking history and COPD, as well as her preference, alcohol septal ablation was chosen. Diagnostic coronary angiography demonstrated an appropriate 1st septal perforator artery and minimal coronary artery disease.
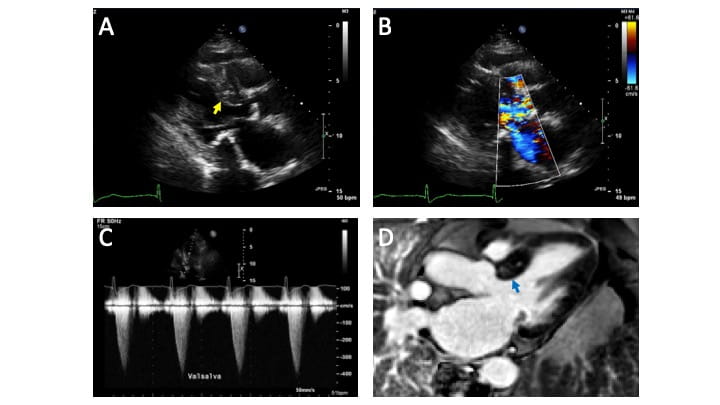
Figure 1. A – Echocardiography in the parasternal long axis view demonstrating severe asymmetric septal hypertrophy of 1.8 cm (yellow arrow). B – Color Doppler echocardiography (same parasternal long axis view as panel A) demonstrating severe outflow turbulence as well as mitral regurgitation. C – Spectral Doppler quantifying severe LVOT obstruction of approximately 64 mm Hg with Valsalva maneuver provocation. D – Cardiac MRI contrast imaging demonstrating mild nonischemic fibrosis in the target region (basal anterior septum) for ablation (blue arrow).
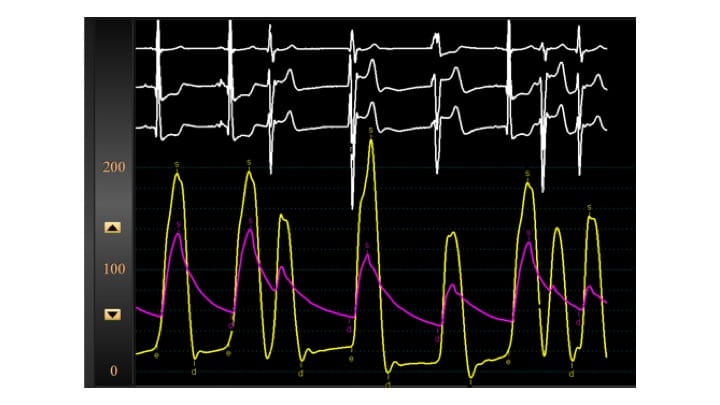
Figure 2. Baseline hemodynamics demonstrating pre-ablation provoked gradient of 100 mm Hg (note wide separation of the left ventricular and aortic pressure curves).
On the day of the procedure, baseline echocardiography again demonstrated severe LVOT obstruction due to septal hypertrophy. Via a right jugular approach, a temporary pacing wire was placed to provide immediate backup should complete heart block occur, and coronary angiography demonstrated the coronary anatomy. (Figure 3, panel A) Initial hemodynamics via left heart catheterization demonstrated a provoked (post-PVC) gradient of 100 mm Hg. (Figure 2) A wire was placed into the 1st septal perforator artery, and an over-the-wire balloon was inflated to isolate the larger branch of the artery. However, a test injection of a mixture of iodinated contrast mixed with microbubble contrast under simultaneous fluoroscopic and echocardiographic visualization demonstrated inadequate coverage of the hypertrophied region. The balloon was then repositioned more proximally to provide adequate coverage of the entire basal septum. (Figure 3, panel B) Echocardiography was performed specifically to rule out extension of the contrast to remote regions of the myocardium. A total of 1.8 mL of ethanol was slowly injected via the inflated over-the-wire balloon. The patient reported mild to moderate angina, which was controlled with medication. Angiograms following the injection demonstrated absent flow in the 1st septal perforator. (Figure 3, panel C)
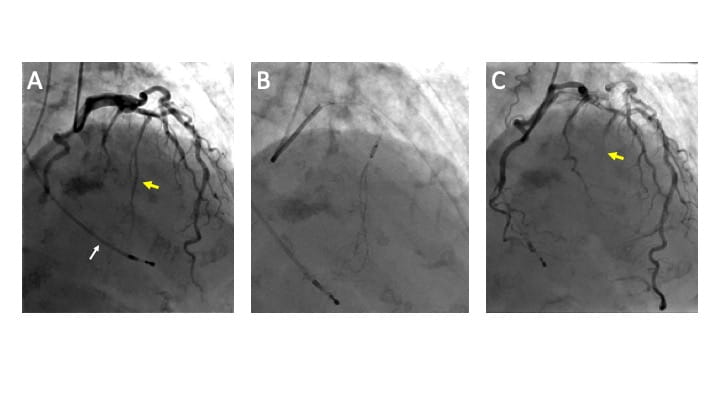
Figure 3. A – Coronary angiogram demonstrating the target 1st septal perforator artery (yellow arrow), and temporary pacing wire in the right ventricle (white arrow). B – Occlusion of the proximal 1st septal perforator artery with an over-the-wire balloon and injection of contrast into the perforator artery. C – Demonstration of lack of flow down the target septal perforator artery (yellow arrow).
Hemodynamics at the conclusion of the ablation demonstrated reduction of the peak provoked gradient down to 15 mm Hg. (Figure 4) The patient was monitored in the ICU for two days, at which point the temporary pacing wire was removed without incident. After two more days of monitoring on the telemetry unit, the patient was discharged home. At one-month office follow-up, the patient reported improving dyspnea on exertion. At three months, follow-up echocardiography demonstrated an approximately five mm reduction in septal thickness, as well as mild residual LVOT gradient of 15 mm Hg and mild to moderate mitral regurgitation. (Figure 5) At one-year follow-up (at the age of 80), her dyspnea on exertion remained improved, and she was pleased with her overall quality of life.
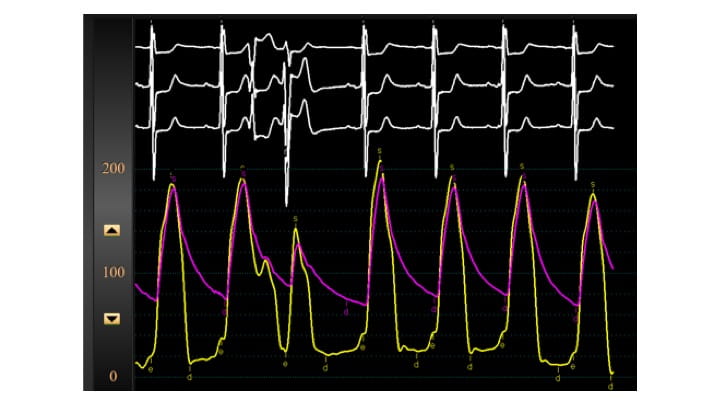
Figure 4. Post ablation hemodynamics demonstrating significant reduced provoked LVOT gradient of 15 mm Hg (note narrow separation of the left ventricular and aortic pressure curves).
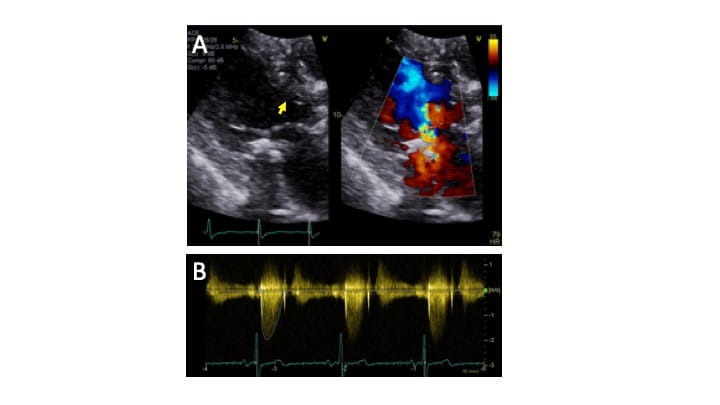
Figure 5. A - Post ablation echocardiography demonstrating thinner basal anterior septum (yellow arrow), with less obstruction and mitral regurgitation by color Doppler. B - Peak LVOT gradient quantified by spectral Doppler (with and without Valsalva maneuver) is decreased to 15 mm Hg.
For HCM patients whose LVOT obstruction is refractory to maximal medical therapy, the choice of septal reduction therapy technique is a complex decision. While septal myectomy surgery is the more established technique, alcohol septal ablation has emerged as a less invasive option in carefully selected patients. In general, younger patients or those with an additional indication for coronary artery bypass or mitral valve repair are considered for septal myectomy, whereas older patients with more risks for cardiopulmonary bypass are considered for septal ablation. In addition, specific attention to the anatomy of the mitral valve apparatus is required given that in certain patients significant redundant mitral tissue is present that may predispose to significant residual obstruction even with aggressive septal reduction. These patients with intrinsic mitral valve derangement are not ideal candidates for alcohol septal ablation.
Several concepts merit further discussion to provide a more nuanced characterization of both types of septal reduction therapy. First, higher volume centers demonstrate improved patient outcomes for both procedures. Mortality, pacemaker implantation risk, and other complications were lowest at the highest volume centers, which represented a minority of the facilities in which these procedures were performed.5 Second, debate has occurred over the impact of introducing a significant amount of scar tissue into the myocardium via the ablation technique. Observational data initially suggested a higher risk of cardiac arrhythmia post-ablation, but more contemporaneous data from separate research groups with long-term follow-up suggest that the risk of adverse outcomes is lower than initially appreciated (and may approximate that of baseline risk).6,7 Finally, a direct comparison of both of these techniques (i.e., a randomized trial) is unlikely to occur. However, recent case-control data comparing outcomes for “matched” patients undergoing each procedure suggest that mortality risk is similar (although the risk of significant residual LVOT gradient and/or re-intervention appears higher with the ablation approach).8
In summary, comprehensive HCM therapy found at high volume centers provides advanced options for the symptomatic patient with LVOT obstruction refractory to maximal medical therapy. A nuanced discussion of advantages and disadvantages of both septal myectomy and alcohol septal ablation is requisite for informed and patient-centered decision making.
The UPMC Hypertrophic Cardiomyopathy Center is the HCM Center of Excellence in western Pennsylvania and offers the entire spectrum of HCM care, from initial diagnosis to multidisciplinary care to genetic counseling. The UPMC Heart and Vascular Institute is leading national research and continues to participate in large multi-center clinical trials, including novel drug development for HCM.
For more information about the Center, or referral of HCM patients, please call the UPMC Hypertrophic Cardio-myopathy Center at 1-877-HCM-UPMC (email HCMCenter@upmc.edu).
1.Gersh BJ, et al. 2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy. Circulation. 2011 Dec 13; 123(24): 2761-96.
2. Wong TC, et al. Novel Pharmacotherapy for Hypertrophic Cardiomyopathy. Cardiology Clinics. 2019; 37(1): 113-17.
3. Smedira NG, et al. Current Effectiveness and Risks of Isolated Septal Myectomy for Hypertrophic Obstructive Cardiomyopathy. Ann Thorac Surg. 2008; 85(1): 127-33.
4. Batzner A, et al. Survival After Alcohol Septal Ablation in Patients with Hypertrophic Obstructive Cardiomyopathy. J Am Coll Cardiol. 2018; 74(24): 3087-94.
5.Kim LK, et al. Hospital Volume Outcomes After Septal Myectomy and Alcohol Septal Ablation for Treatment of Obstructive Hypertrophic Cardiomyopathy: US Nationwide Inpatient Database, 2003-2011. JAMA Cardiol. 2016; 1(3): 324-32.
6.Jensen MK, et al. Alcohol Septal Ablation in Patients With Hypertrophic Obstructive Cardiomyopathy: Low Incidence of Sudden Death and Reduced Risk Profile. Heart. 2013; 99: 1012-17.
7.Veselka J, Krejci J, Tomasov P, Zemanek D. Long-term Survival After Alcohol Septal Ablation for Hypertrophic Obstructive Cardiomyopathy: A Comparison With General Population. Eur Heart J. 2014; 35: 2040-45.
8.Nguyen A, et al. Surgical Myectomy Versus Alcohol Septal Ablation for Obstructive Hypertrophic Cardiomyopathy: A Propensity Score-Matched Cohort. J Thorac Cardiovasc Surg. 2019; 157(1): 306-15.